Solian
Roger Skebelsky, PA-C, BSN, RN - Department of Emergency Medicine
- Mount Sinai Hospital
- Chicago, IL
Buy solian 50mg onlineA mixture preparation containing 500 mg of dilox anide furoate and 400 mg of metronidazole has been mar keted as "Entamizole" by Abbott in India medicine definition purchase solian 50 mg with mastercard. Entamizole is on the market as an offwhite symptoms yeast infection men buy generic solian 100mg on-line, pillowshaped treatment pancreatitis cheap 100 mg solian overnight delivery, filmcoated tablet treatment zenkers diverticulum solian 50 mg fast delivery, and as a pinkcolored syrupy suspension with fruit taste. Each 10 ml of suspension contains 250 mg of diloxanide furoate and 200 mg of metronidazole. Those requiring altered dosages the only contraindication to the use of diloxanide furoate is a hypersensitivity to this drug. Bioavailability the gastrointestinal absorption of diloxanide, the luminally energetic drug, is lowered when administered because the furoate ester formulation, subsequently resulting in higher concentrations of the prodrug diloxanide furoate within the bowel lumen for a longer time frame (Botero, 1964; Wolfe, 1973). In the intestine, diloxanide furoate is basically, if not fully, hydrolyzed into diloxanide and furoic acid beneath the mixed action of bacterial and gut esterases (Osisanya, 1986; Gadkariem et al. It is the remaining 10% of unabsorbed diloxanide that persists in the gut lumen that exerts the antiamebic exercise of the drug. Adults A 10day course comprising one 500mg pill 3 times per day is beneficial for the therapy of asymptomatic E. In general, the really helpful dose and duration of therapy are the identical, whether or not the drug is used alone for asymptomatic cyst passers or at the facet of other ther apy for symptomatic or invasive disease. Drug distribution After absorption of an estimated 90% of the hydrolyzed diloxanide, it is very quickly conjugated to a glucuronide. It is thus current in peripheral blood virtually wholly as its glucu ronide conjugate, with solely ~ 1% remaining in plasma as free diloxanide. Excretion After hydrolysis, absorption, and glucoronidation of diloxa nide furoate, the glucuronide conjugate is rapidly excreted within the urine (Dubey et al. There is a few proof that acti vated charcoal could also be useful in controlling this effect (Felix et al. The solely different vital side effects embrace nausea in approximately 5%, anorexia in approxi mately 3%, and diarrhea and abdominal cramping, each occurring at a frequency of roughly 2% (Wolfe, 1973). Risk in being pregnant the safety of diloxanide furoate during pregnancy and lacta tion has not been established, and use throughout these intervals ought to due to this fact be prevented. A comparable lack of embryotoxicity was reported in rats given the identical dose from the days 1�20. Treatment of asymptomatic Entamoeba histolytica cyst excretion Amebiasis is an infection attributable to the protozoal organism E. It is still not clear whether all noninvasive asymptomatic infections should be treated (Farthing, 2006). Others argue that, given the potential for progression to invasive illness, all individuals with asymptomatic infection ought to be treated regardless. Outbreaks of refractory intestinal amoebiasis inside institutional care facilities have been successfully managed by treatment of asymptomatic carriers of E. Amebicides are categorized according to their web site of action, which could be in the bowel lumen, mucosa, and/or extraintestinal tissues. Diloxanide furoate is certainly one of the three principal luminal amebicides that exhibit little or no activity within the bowel wall or extraintestinal tissues. Of the luminal amebicides, diloxanide furoate was favored for a few years due to its safety and effectiveness, with cure charges in asymptomatic carriers exceeding 90% (Woodruff and Bell, 1960; Forsyth, 1962; Wilmot et al. Although this study centered on phar macologic properties of the conjugated drug, it may lead to new preparations of diloxanide furoate that could overcome a variety of the shortcomings of the drug in contrast with paro momycin. Iodoquinol (diiodohydroxyquin), one other luminal agent, can be well tolerated, but requires 20 days of therapy 3198 Diloxanide Furoate and is contraindicated in sufferers with hepatic failure or allergy to iodine (Reed, 1992; see Chapter 191, Iodoquinol and quinacrine). Con-A conjugated mucoadhesive microspheres for the colonic delivery of diloxanide furoate. First molecular identification of Entamoeba moshkovskii in human stool samples in Tunisia. Difficulties in the therapy of intestinal amoebiasis in mentally disabled people at a rehabilitation institution for the intellectually impaired in Japan. Stability research on diloxanide furoate: effect of pH, temperature, gastric and intestinal fluids. Experiences with the utilization of metronidazole within the remedy of non dysenteric intestinal amoebiasis. Controlled open investigation of diloxanide furoatemetronidazole in intestinal amoebiasis. Treatment of invasive or extraintestinal amebiasis In acute amebic dysentery or extraintestinal illness, diloxa nide furoate is insufficient as a single agent for remedy. It should be used both concurrently with, or subsequent to , different agents that have exercise in the bowel wall and tissues, particularly the liver. In basic, a nitroimidazole (either metronidazole [see Chapter 99, Metronidazole] or tinidazole [Chapter one hundred, Tinidazole]) is given initially, as a outcome of these medication possess activity in all tissues. A luminal amebicide such as diloxanide furoate is then administered to eradicate intes tinal an infection, regardless of whether the organism is discovered in the stool, as a end result of nitroimidazoles have restricted activity against cysts and are almost completely absorbed from the small bowel. These medication, due to this fact, have an impact as intralu minal amebicides provided that the organisms are in shut proximity to the colonic mucosa (Kanani and Knight, 1972; Powell et al. In numerous studies, the coformulation of metronidazole with diloxa nide furoate (Entamizole) has been shown to be effective when used in a shorter 5day course (Latonio, 1987; Qureshi et al. Treatment of giardiasis Combined preparations of diloxanide furoate and metroni dazole have additionally been shown to be efficient within the remedy of giardiasis (Qureshi et al. Blastocystis hominis and irritable bowel syndrome Diloxanide furoate, used at the side of trimethoprim� sulfamethoxazole and secnidazole, was shown in a small pilot research to be moderately efficient for the clearance of B. Clinical trials with entamide furoat, entamide piperazine sulphate and emetine bismuth iodide. Clinical pilot research: efficacy of triple antibiotic therapy in Blastocystis optimistic irritable bowel syndrome patients. Mass an infection with Entamoeba histolytica in a Japanese institution for people with psychological retardation: epidemiology and control measures. Comparative in vitro activity of mefloquine, diloxanide furoate and different conventionally used amoebicides in opposition to Entamoeba histolytica. Efficacy of a combined diloxanide furoate�metronidazole preparation within the remedy of amoebiasis and giardiasis. Comparative trial of metronidazole against a mixture of dehydroemetine, tetracycline, and diloxanide furoate. Susceptibility of an emetineresistant mutant of Entamoeba histolytica to a quantity of medication and to channel blockers. Problems in recognition and prognosis of amebiasis: estimation of the worldwide magnitude of morbidity and mortality. Iodoquinol displays energetic amebicidal activity inside the lumen of the gut against the cyst section of E. For Dientamoeba fragilis, a minimum amebicidal focus of 128 �g/ml seems to be necessary to guarantee efficacy (Chan et al. Although there are few information out there concerning resistance to iodoquinol, Samuelson et al. The usual dose of iodoquinol for adults is 650 mg 3 times every day for 20 days, and for kids the dose is 30�40 mg/kg/day in three divided doses (to a most of 1950 mg/day) for 20 days.
Cheap solian 50mg on-lineA proposed mechanism of resistance could also be Pglycoprotein�mediated drug efflux pumps treatment zenkers diverticulum generic solian 50 mg amex, which remove hydrophobic medicine from the parasite cytosol medicine man dr dre discount 50 mg solian otc. The emetine resistance was in a position to medicine encyclopedia purchase solian 100mg on line be reversed in vitro by calcium and sodium channel blockers medicine 600 mg cheap solian 100mg online, which are known to inhibit the perform of Pglycoprotein transporters (Samuelson et al. Since the identification of other morphologically similar however nonpathogenic species of Entamoeba, particularly E. This routine has additionally been proven to be efficient for amebic liver abscess (Powell et al. Doses of 25 mg/kg every day in three divided doses for 10 days have also been used (Botero, 1964; Rubidge et al. Elsewhere the drug is on the market by way of numerous special access schemes or via specialist compounding pharmacies. Children are more likely to expertise unwanted effects and should be monitored intently for visible modifications (see section 2, Toxicity and medical uses of the drug). Treatment of a series of sufferers with belly pain related to carriage of D. Although toxicity is unusual when iodoquinol is used at recommended doses (Chemotherapy of protozoal infections, 2008), a key limiting function of the drug has been its toxicity profile, which includes optic neuritis and peripheral neuropathy. The main critical side effect described is subacute optic neuritis and optic atrophy amongst iodoquinol recipients, with the frequency possibly increasing after extended use (Chemotherapy of protozoal infections, 2008). Peripheral neuropathy has also been reported (Chemotherapy of protozoal infections, 2008), as have seizures in youngsters (Fisher et al. Because iodoquinol accommodates iodine, it could have an effect on measurements of thyroid function, and it must be used with warning in patients with preexisting thyroid dysfunction and prevented in sufferers with iodine allergy (Moon and Oberhelman, 2005). Other widespread unwanted facet effects embrace nausea and vomiting, which may be restricted by ensuring sufficient hydration and regular small meals, in addition to skin rash, urticaria, and/or pruritus, headache, and perianal itch (Moon and Oberhelman, 2005). Although efficient, because of the potential unwanted effects the utilization of iodoquinol is now limited to second-line remedy for eradication of intraluminal cysts of E. Because iodoquinol is energetic only in the intestine lumen, it has no position in the therapy of amebic liver abscess or other invasive forms of amebic an infection, which must be treated with an imidazole such as metronidazole (see Chapter 90, Metronidazole) (Jong, 2002). Likewise, in observational studies iodoquinol has not demonstrated usefulness for treatment of Blastocystis infection (Roberts et al. More lately, it has been used as an antiprotozoal agent because it has exercise in opposition to G. Quinacrine has additionally been used for the remedy of tapeworm, however it has been changed for this indication by newer, less poisonous agents. H3C H3 C N the recommended dose of quinacrine for giardiasis is 300 mg orally per day in three divided doses, for 5�7 days. For children, the really helpful dose is 2 mg/kg body weight three times per day for 5�7 days. There are insufficient information to information dose adjustment in patients with renal or hepatic impairment, or in obese sufferers. Quinacrine is quickly absorbed from the gastrointestinal lumen, with a time to peak focus of 1�3 hours, though its bioavailability is unquantified (Sanofi, 1995). It has a large volume of distribution and is slowly excreted by the kidney, taking up to months to be fully excreted (Mepacrine hydrochloride, 1995). Quinacrine is not used for the prophylaxis and treatment of malaria because of its toxicity profile (Looareesuwan et al. In latest research, it has been proposed as salvage remedy for refractory giardiasis (M�rch et al. There has also been a renewed curiosity in the usage of quinacrine due to its exercise against prions, corresponding to these causing Creutzfeldt�Jakob illness. Quinacrine has also been used as a pleural sclerosing agent for malignant and nonmalignant pleural effusions (Dikensoy and Light, 2005), as well as an agent in nonsurgical sterilization (Sokal et al. Compassionate use of quinacrine in Creutzfeldt-Jakob illness fails to present vital results. Mepacrine accumulation throughout therapy of chloroquine-resistant falciparum malaria. Dientamoeba fragilis, a protozoan parasite in grownup members of a semicommunal group. Increased incidence of nitroimidazole-refractory giardiasis on the Hospital for Tropical Diseases, London: 2008�2013. Safety of quinacrine contraceptive pellets: outcomes from 10-year follow-up in Vietnam. Benznidazole was beforehand marketed underneath the names Rochagan, Ragonil, and Radanil, and till just lately was manufactured by Hoffman La-Roche. Routine susceptibility Benznidazole is energetic against both the extracellular trypomastigote and intracellular amastigote stage of T. It additionally possesses some in vitro antibacterial exercise, particularly in opposition to anaerobic and microaerophilic micro organism (Hof, 1989). However, the numerous toxicity associated with using benznidazole (see below underneath 6, Toxicity), along with the provision of quite a few different treatment choices, has restricted the utilization of benznidazole as an antibacterial agent in scientific follow. Emerging resistance and cross-resistance Treatment failure in Chagas illness is thought to be due in part to benznidazole-resistant T. As demonstrated by Mejia and colleagues, in vitro benznidazole resistance can additionally be readily induced. Benznidazole resistance can also be related to resistance to alternate trypanocidal brokers (Filardi and Brener, 1987). These drug-resistant clones seem to be as virulent, inflicting comparable ranges of parasitemia and mortality to wild-type strains (Murta and Romanha, 1998). Despite the increasing understanding of the mechanisms of benznidazole resistance, the correlation between in vitro sensitivity testing and clinical consequence may not be dependable. Newborn infants and kids the really helpful dose for youngsters younger than 12 years is 5�7. In vitro synergy and antagonism As discussed in Chapter 193, Nifurtimox, in vitro research have demonstrated that the amino acid analog buthionine sulfoximine increases the trypanocidal activity of benznidazole (Foundez et al. Combination remedy with benznidazole and ketoconazole in mice has been shown to increase trypanocidal activity against T. This could clarify why therapy with benznidazole is more practical in acute infection, when cytokine levels are more likely to be at their highest. Unlike nifurtimox, the technology of nitroanion radicals with subsequent manufacturing of toxic decreased oxygen metabolites performs only a minor position within the trypanocidal effect of benznidazole (Castro et al. No information can be found regarding dose adjustment in sufferers with vital hepatic or renal dysfunction. The pharmacokinetics of benznidazole was best described by a one-compartment linear mannequin with first-order absorption and first-order elimination.
Discount 100mg solian mastercardHowever treatment 2 stroke buy 50mg solian with amex, at a inhabitants degree medicine head buy solian 100mg free shipping, higher mefloquine concentrations are associated with improved clinical outcomes medicine journals impact factor solian 100 mg on-line. For patients receiving mefloquine prophylaxis treatment yeast infection generic 100 mg solian visa, prophylaxis failure is defined by a confirmed P. Excretion In people, mefloquine appears to be metabolized by cytochrome P450-3A4 to one acid and one alcohol metabolite (Fontaine et al. In vivo, the hydroxyl metabolite is current only in hint quantities and the carboxylic acid metabolite predominates (Schwartz et al. Considering the wide selection of lumefantrine plasma levels observed clinically during initiation of artemether�lumefantrine therapy, the importance of this interaction is doubtful, especially if lumefantrine is given with meals, which considerably increases bioavailability. Confirmation of this interaction in falciparum malaria patients who might have gastrointestinal disturbances and altered absorption is required. Co-artemether could additionally be safely used after failure of antimalarial prophylaxis or remedy with mefloquine (see Chapter 169, Artemisinins). Plasma drug level measurements revealed adequate systemic exposure to artemether, dihydroartemisinin, lumefantrine, and mefloquine, well according to the medical setting. Mefloquine may lower effective concentrations of antiepileptic medicines, so their dosages could must be adjusted while a affected person is taking mefloquine (Roche, 2003a). Of observe, mefloquine is contraindicated in seizure issues (see part 6a, Neurologic and neuropsychiatric toxicity). Concurrent co-administration of mefloquine with the live typhoid vaccine Ty21A could result in a decreased immunologic response to the vaccine. Therefore vaccinations with attenuated stay micro organism must be accomplished no much less than 3 days before the first dose of mefloquine (Roche, 2003a). The Mephaquin product label recommends that the potential results of mefloquine on drugs being taken concurrently, in particular anticoagulants and antidiabetic medicine, be clarified earlier than travel (Verdun, 2006). However, mefloquine can be related to extra frequently observed neurologic and gastrointestinal results which have practical consequences for the clinical use of the drug, and differing significance relying on the clinical context. The neurologic results of mefloquine have obtained a lot public attention and litigation. Mefloquine has been related to larger rates of sure gastrointestinal and neurologic effects. These have been reviewed extensively elsewhere (Taylor and White, 2004); the salient factors are summarized here. The incidence of several gastrointestinal and neurologic results is greater than with comparator medicine corresponding to chloroquine (dizziness), halofantrine (nausea, vomiting, fatigue, and dizziness), and lumefantrine (nausea, vomiting, dizziness, and insomnia) (Kofi Ekue et al. Of note, these results are additionally attributable to malaria, but have been attributed to mefloquine in two-thirds of cases (Taylor and White, 2004). Such antagonistic effects (vertigo 96%, nausea 82%, and headache 73%) have been additionally observed at excessive frequency when mefloquine was given at therapeutic doses to healthy volunteers in a medical examine to assess its tolerability in the context of emergency standby remedy (Rendi-Wagner et al. However, in the context of treatment of malaria, probably the most clinically vital antagonistic occasion is gastrointestinal misery (vomiting), particularly in youngsters. It is important to notice that this will likely lead to incorrect dosage and scientific failures (Nosten and White, 2007). This impact is age associated, with an incidence as high as 30% in infants younger than 2 years (Taylor and White, 2004). Gastrointestinal misery could be considerably decreased if the drug is given as a split dose or day by day for three days in a fixed-dose mixture (Simpson et al. Severe neurologic results (discussed in part 6a, Neurologic and neuropsychiatric toxicity) might happen in as many as 5% of patients recovering from cerebral malaria after mefloquine therapy (Nguyen et al. Neurologic and neuropsychiatric toxicity Travelers taking malaria chemoprophylaxis characterize an otherwise healthy inhabitants among whom the neurologic results of mefloquine are of some concern. However, mefloquine is less nicely tolerated in terms of neuropsychiatric antagonistic occasions than comparator drugs, and neuropsychiatric opposed events occur with higher frequency. The information that help these assertions have been accrued in a series of five double-blind, randomized, controlled research in which the neuropsychiatric effects of mefloquine relative to 6. This research used a questionnaire by which the next attainable neurologic antagonistic occasions have been solicited: headache, unusual or vivid goals, dizziness, anxiety, depression, visible disturbance, fits, or seizures. This research reported the incidence of neurologic events classified in terms of any, average, and severe neurologic antagonistic effects (Table 178. Mefloquine therapy was associated with a better incidence of average neurologic opposed events (p = zero. The incidence of any neuropsychiatric occasion was 14% within the atovaquone�proguanil arm and 29% in the mefloquine arm (p = zero. Thus the net mefloquine-attributable incidence rates for common and treatment-limiting neuropsychiatric opposed events were 15% and 3. A larger fee of vivid goals was observed within the loading dose arm relative to chloroquine (13% vs. Thus the incidence of neuropsychiatric events attributable to mefloquine was between 5. Two individuals in the mefloquine arm have been withdrawn from treatment owing to depression and suicidal thoughts. On closer scrutiny of the records, each people had preexisting psychiatric situations. Thus the net incidence of mefloquine-attributable neuropsychiatric antagonistic events was 17%. No drug-attributable treatment-limiting adverse events had been reported on this research. Sustained attention, coding speeds, and visuomotor performance were examined using validated computerized checks. These results had been extra prevalent earlier in prophylaxis and in people with lower physique weight (mostly women). There had been no vital variations between the therapy groups in phrases of neurologic function. There have been a variety of stories of severe life-threatening psychiatric events, including suicide and a quantity of murder (Cameron Ritchie et al. However, in a quantity of bigger, retrospective, database research, the incidence of such occasions has been estimated at between 1/607 and 1/20,000 (Chen et al. The incidence of similar results among those taking chloroquine is 1/1181 to 1/13,600 (Chen et al. However, the true mefloquine-attributable threat of such results is difficult to discern because of inherent methodologic flaws in such research, together with pattern bias and a lack of goal compliance information. Overall, severe neurologic results might occur in as many as 5% of patients recovering from cerebral malaria after mefloquine remedy, and hence many authorities now urge warning in the utilization of mefloquine in patients recovering from extreme malaria (Nguyen et al. Incidence of neurologic adverse occasions (percentage of people affected) in non-immune vacationers taking completely different regimens for malaria prophylaxis.
Purchase solian 50 mg with visaAn experimental mannequin for the research of potential mechanisms of drug resistance in acute T treatment 5th toe fracture discount solian 100 mg. However treatment low blood pressure purchase solian 100mg free shipping, this check is sluggish and labor intensive medications jokes solian 50 mg line, and is on the market solely in specialised analysis laboratories symptoms zoloft purchase 100 mg solian. This variability occurs in isolates from sylvatic reservoirs and vectors in addition to in isolates from populated areas the place nifurtimox is used in the remedy of Chagas illness. Furthermore, ranges of intracellular free thiols negatively correlate with susceptibility of a quantity of T. Acute (first-stage) infection involves the bloodstream and lymphatic system, and infrequently goes unrecognized. However, some patients develop a noticeable lesion at the web site of the bite (trypanosomal chancre). Second-stage an infection is characterized by development of progressive neurologic dysfunction brought on by central nervous system invasion by the parasite, and is uniformly fatal with out remedy. In vitro synergy and antagonism Although nifurtimox is at present used in combination with other antitrypanosomal agents for therapy of T. As with different nitro-containing drugs, the metabolic reduction of the nitro group is considered to be crucial for the exercise of nifurtimox, in addition to being liable for lots of its opposed results. Until lately, it was believed that trypanosomes are comparatively deficient in enzymes to shield themselves in opposition to "oxidative stress" generated by reactive oxygen species. This work has indicated that alkylation of mobile macromolecules by metabolites of nifurtimox and depletion of intracellular ranges of thiol "scavenger molecules". Marked morphologic adjustments and lowered numbers of bloodstream trypomastigotes could be demonstrated shortly after the administration of nifurtimox (Haberkorn and G�mnnert, 1972). While the manufacturer recommends a three times a day dosing routine, each twicedaily or 4 occasions a day regimes are beneficial by some authorities. For adults (and children 17 years of age) the dose really helpful by the producer is 8�10 mg/kg/day in three divided doses. The manufacturer recommends that the dose be administered to breastfeeding infants and young children by pulverizing the tablets and mixing the powder with a small amount of food, which is then given before the meal (Lampit Product Information). No particular data is out there on the usage of nifurtimox in untimely neonates. Bioavailability Pharmacokinetic research of radiolabeled nifurtimox in rats and canines recommend that the drug is nicely absorbed from the gastrointestinal tract following oral administration (Duhm et al. Following the administration of a single 15-mg/kg oral dose of nifurtimox to healthy human volunteers, the Cmax was zero. When administered at a dose of 5 mg/kg three times every day, serum levels of nifurtimox three hours following ingestion of the dose had been between zero. Nifurtimox seems in breast milk following the administration of the drug to lactating animals (Castro et al. Drug distribution Relatively low concentrations of nifurtimox seem within the plasma of rats and canines and the serum of people following oral administration, presumably as a end result of in depth firstpass hepatic metabolism (Duhm et al. The focus of nifurtimox in numerous tissues following oral administration in these research was also relatively low (Duhm et al. It is unknown whether delayed clearance of nifurtimox in these sufferers was due to reduced hepatic metabolism or impaired renal excretion of the drug. The manufacturer recommends that nifurtimox "not be given to patients with renal failure because of the lack of information in this affected person group" (Lampit Product Information). Adverse reactions and toxicity 3215 (10�100 �M), and host cell toxicity is seen at concentrations above 100 �M (Bock et al. Although these data suggest that nifurtimox demonstrates concentration-dependent activity in vivo, no medical studies have been performed to verify this. Excretion Less than 1% of an orally administered dose of nifurtimox appears unchanged in urine, suggesting intensive hepatic metabolism previous to excretion (Medenwald et al. Animal studies of radiolabeled nifurtimox counsel that metabolites of nifurtimox are excreted within the urine (Duhm et al. Drug interactions No information is on the market concerning the interaction of nifurtimox with other medication or meals. However, intolerance to alcohol has been described in sufferers taking nifurtimox, and the product information states that "it is suggested to completely avoid the ingestion of alcoholic beverages in order to stop attainable side-effects" (Lampit Product Information). Preclinical toxicity studies this topic has been beforehand summarized in some detail (Castro et al. In animal fashions, toxicity appears to affect tissues that are considered most susceptible to the results of reactive oxygen species, such because the brain and testes. For instance, central nervous system symptoms (which have been typically reversible) had been widespread in rats and dogs given excessive doses of nifurtimox (100 mg/kg) over an extended period of time, and had been related to spongiosis, glial cell proliferation, and decreased neuron counts. Similar doses also resulted in testicular atrophy and full inhibition of spermatogenesis in rats (Hoffman, 1972). It has been shown that nifurtimox is metabolized within the rat heart, leading to biochemical and cellular adjustments which will doubtlessly contribute to impaired cardiac an infection in chronic Chagas illness (Bartel et al. Nifurtimox has also been related to increased tumorigenesis and carcinogenesis in rats and rabbits (Steinhoff and Grundmann, 1972; Teixeira et al. Clinical toxicity the paucity of well-designed potential placebo-controlled randomized clinical trials of using nifurtimox, and the suboptimal documentation of adverse results in scientific research when nifurtimox has been in contrast with placebo, make it difficult to determine whether antagonistic results reported with the use of the drug are brought on by the drug or by the an infection itself. In early evaluations of nifurtimox in acute and persistent Chagas disease, opposed effects had been documented in as a lot as two-thirds of children and adolescents and in as a lot as 85% of adults (Wegner and Rohwedder, 1972a; Wegner and Rohwedder, 1972b; Castro, 2000). The commonest adverse results described in these research have been gastrointestinal, and included anorexia, nausea, vomiting, stomach pain, and weight reduction. In addition, central nervous system signs, including "nervous excitation," psychosis, vertigo, seizures, insomnia, and paresthesia, were additionally comparatively common. Peripheral neuropathy can be common, and seems to be related to the cumulative dose of the drug (Brener, 1979). Permanent discontinuation of drug administration owing to opposed effects was required in a significant proportion of these sufferers, including one-quarter of adults (Wegner and Rohwedder, 1972a; Wegner and Rohwedder, 1972b; Castro, 2000; Castro et al. In a retrospective uncontrolled study the place the efficacy and antagonistic impact profile of nifurtimox and benznidazole were compared in Brazilian adults with persistent Chagas disease, extreme opposed results requiring discontinuation of the drug were documented in 6/8 (75%) patients who obtained nifurtimox compared with 17/41 (42%) of patients receiving benznidazole, suggesting that nifurtimox is less properly tolerated than benznidazole, a minimal of in this affected person group (Levi et al. Significant adverse results (including weight reduction and neurological, constitutional, musculoskeletal, or dermatological effects) were additionally reported with the utilization of nifurtimox in a cohort of grownup Latin American migrants being handled in Switzerland, resulting in a therapy cessation price of forty three. Although little is understood about the effects of long-term nifurtimox remedy on hepatic, renal, and hematopoietic perform, elevation in liver enzymes or bilirubin and suppression of bone marrow function were documented in earlier scientific trials in Chagas illness, and have been sometimes reported subsequently (Wegner and Rohwedder, 1972a; Wegner and Rohwedder, 1972b; Jackson et al. Hemolytic 3216 Nifurtimox anemia, in affiliation with glucose-6-phosphate dehydrogenase deficiency, has been described (Van Voorhis, 1990). An elevated price of nonrandom chromosomal abnormalities has been described in youngsters receiving nifurtimox for the remedy of Chagas disease (Gorla et al. Although the potential use of drug delivery nanoparticles to improve the efficacy and potentially scale back the toxicity of nifurtimox has been investigated (Gonzalez-Martin et al. For sufferers aged over 50 years, the authors suggest that treatment should be thought of elective (Bern et al. Treatment of Chagas disease Nifurtimox is at present licensed to be used in several Latin American countries for the treatment of Chagas illness. Nifurtimox is taken into account second-line remedy to benznidazole (see Chapter 192, Benznidazole) within the remedy of Chagas illness in many countries, primarily due to its adverse impact profile. Several critiques of the use of antitrypanosomal drug remedy in Chagas disease have been revealed over the past 30 years (Brener, 1979; Marr and Docampo, 1986; Coura, 1996; Cerecetto and Gonzalez, 2002; Bern et al.
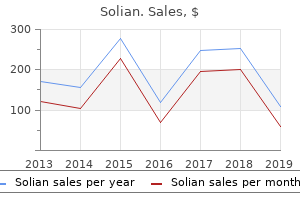
Solian: 100 mg, 50 mg
Generic solian 100 mg on lineMechanism of action of antifungal medication medications listed alphabetically proven solian 100 mg, with special reference to the imidazole derivatives symptoms ketosis discount solian 50mg. Hyalohyphomycosis by Paecilomyces lilacinus in a renal transplant affected person and a evaluate of human Paecilomyces species infections medications harmful to kidneys cheap solian 100 mg line. Human pharmacology of griseofulvin: the effect of fat intake on gastrointestinal absorption symptoms 7 days before period generic 50mg solian with amex. A population-based case� management examine of oral griseofulvin treatment during being pregnant. Therapeutic choices for the treatment of tinea capitis: griseofulvin versus fluconazole. Terbinafine hydrochloride oral granules versus oral griseofulvin suspension in youngsters with tinea 7b. Tinea corporis, tinea cruris, and tinea pedis Tinea corporis is usually efficiently managed with topical remedy. Topical griseofulvin can be utilized, and a number of formulations have been described (Kassem et al. Systemic treatment with griseofulvin is indicated for widespread illness or when granulomatous lesions occur. Tinea cruris and tinea pedis are additionally typically managed with topical antifungal agents, with griseofulvin reserved for resistant cases. Symptomatic enchancment could take as a lot as 6 weeks and scientific treatment might not occur before 6 months. Terbinafine a hundred twenty five mg twice daily was discovered to be considerably superior to griseofulvin 250 mg twice every day for chronic moccasin-type tinea pedis with mycologic cure rates of 88% and 45%, respectively (Savin, 1989; Hay et al. Onychomycosis and other uses the clinical utility of griseofulvin for the remedy of onychomycosis is restricted by the requirement for prolonged therapy, and griseofulvin is at present not really helpful by most authorities. Relatively poor response charges could also be due to suboptimal penetration of drug into the nail (Weitzman and Summerbell, 1995). Although the preliminary response rates of griseofulvin and terbinafine could additionally be related, griseofulvintreated sufferers have the next rate of relapse (Hofmann et al. A renal transplant patient 2932 Griseofulvin capitis: results of two randomized, investigator-blinded, multicenter, worldwide, controlled trials. Griseofulvin versus terbinafine within the treatment of tinea capitis: a meta-analysis of randomized, scientific trials. Meta-analysis of randomized, managed trials evaluating particular doses of griseofulvin and terbinafine for the therapy of tinea capitis. Isolated erythroid hypoplasia and renal insufficiency induced by long-term griseofulvin therapy. Absorption, distribution, metabolism, and excretion of griseofulvin in man and animals. A randomized, doubleblind, parallel-group, duration-finding study of oral terbinafine and open-label, high-dose griseofulvin in children with tinea capitis because of Microsporum species. Studies in the biochemistry of micro-organisms: griseofulvin, C(17)H(17)O(6)Cl, a metabolic product of Penicillium griseo-fulvum Dierckx. Comparison of in vitro activities of voriconazole and 5 established antifungal agents towards different species of dermatophytes utilizing a broth macrodilution technique. Successful therapy of chronic tinea pedis (moccasin type) with terbinafine (Lamisil). Intracutaneous distributions of fluconazole, itraconazole, and griseofulvin in Guinea pigs and binding to human stratum corneum. Meta-analysis of randomized, controlled trials evaluating griseofulvin and terbinafine within the treatment of tinea capitis. Clinical efficacy and tolerability of terbinafine (Lamisil)-a new topical and systemic fungicidal drug for treatment of dermatomycoses. A double blind examine of itraconazole vs griseofulvin in patients with tinea pedis and tinea manus. The effect of griseofulvin on the gene regulation of beta-tubulin in the dermatophyte pathogen Trichophyton rubrum. Haloprogin was synthesized in 1963 by Seki as one of a collection of halophenol-iodopropargyl ethers with antifungal activity (Seki et al. Haloprogin is most commonly administered as 1% cream, during which the lively drug is mixed with polyethylene glycol 400, polyethylene glycol 4000, diethyl sebacate, and polyvinylpyrrolidone. Haloprogin can be formulated as an answer, by which the lively compound is dissolved in alcohol and diethyl sebacate. Routine susceptibility Standardized susceptibility testing methodology for haloprogin has not been developed. In Can dida albicans, haloprogin causes decreased oxygen uptake, decreased 14C-L-leucine incorporation, and intracellular potassium loss, probably suggesting lack of cell membrane integrity (Harrison and Zygmunt, 1974). Haloprogin does appear to be safe in younger children and has been administered longterm to a 4-year-old youngster without any clinical or biochemical evidence of toxicity (Hughes et al. In humans haloprogin is well tolerated, however once in a while causes delicate skin irritation (Hermann, 1972a). There was no proof of allergic manifestations in early testing, but several instances were subsequently reported (Rudolph, 1975). One of those cases occurred with haloprogin solution, and the stabilizing and solubilizing agent diethyl sebacate was implicated (Berlin and Miller, 1976). Pregnant and lactating mothers the safety of haloprogin in pregnancy has not been established. Haloprogin is more practical than placebo (vehicle) for the therapy of dermatophytoses (Katz and Cahn, 1972) and is effective as 1% tolnaftate for the therapy of tinea pedis and different dermatophyte infections (Carter, 1972; Hermann, 1972a). Haloprogin was inferior to clotrimazole 1% for the treatment of tinea cruris in army personnel (Van Dersarl and Sheppard, 1977). Haloprogin 1% is as efficient as topically utilized nystatin ointment for the remedy of cutaneous candidiasis with response charges in extra of 82% (Carter and Olansky, 1974). Haloprogin has subsequently been demonstrated to be effective for the remedy of cutaneous candidiasis. Haloprogin has also been used to treat a 4-year-old lady with chronic mucocutaeous candidiasis over a 3-year period. This protracted course of therapy was not related to any medical or laboratory abnormalities (Hughes et al. The range of systemic absorption in laboratory animals following topical software ranges from 19% to one hundred pc (Weikel and Bartek, 1972). Studies utilizing various formulations of 14C-haloprogin in people demonstrate that 9�15% of the applied compound is absorbed and subsequently excreted in the urine (Hermann, 1972a). Drug distribution the distribution of haloprogin has been studied in laboratory animal models. The major metabolite is 2,4,5-trichlorphenol, which is excreted within the urine (Weikel and Bartek, 1972). Laboratory evaluation of M-1028 (2,4,5-trichlorophenyl gamma-iodopropargyl ether), a brand new antimicrobial agent. The antimalarial properties of artemisinin were first reported within the Western literature in 1979 (Jiang et al. Extensive clinical research have demonstrated that these drugs have a highly potent antimalarial exercise, which, mixed with a broad stage specificity of motion, results in a faster scientific and parasitologic response than some other antimalarial agent in scientific use (Hien and White, 1993).
Syndromes - Stroke or transient ischemic attack (TIA)
- Emulsoil
- Insoluble fiber is found in foods such as wheat bran, vegetables, and whole grains. It adds bulk to the stool and appears to help food pass more quickly through the stomach and intestines.
- Cough with blood
- Follow a low-salt diet, which may reduce fluid buildup and swelling.
- Vitamin B6
- You have questions or concerns about the vaccine
- Selenium
Generic 50 mg solian with visaRelapse of meningitis was reported in 4 patients (15%) receiving placebo versus none receiving fluconazole medications not to take after gastric bypass generic solian 50 mg without a prescription. When in contrast with itraconazole (200 mg daily) medicine 7 day box discount solian 100mg without prescription, fluconazole (200 mg daily) was associated with considerably fewer relapses (4% vs medicine zocor order 50 mg solian with visa. Blastomycosis Several open-label trials have investigated the utility of fluconazole for the treatment of blastomycosis treatment myasthenia gravis purchase 50mg solian free shipping. Although the outcomes demonstrated efficacy, the rate of response was lower than that observed in comparable studies with itraconazole. Options for treatment of blastomycosis embody amphotericin B, ketoconazole, itraconazole, and fluconazole, although comparative studies are lacking. Fluconazole is suggested in its place for delicate to reasonably severe cases in patients unable to tolerate itraconazole. A multicenter, randomized trial of 23 patients in contrast fluconazole dosed at 200�400 mg every day and documented success in 62% and 70% of patients, respectively (Pappas et al. Another multicenter, randomized, research of 39 sufferers investigated larger dosages of oral fluconazole and located dosages of four hundred and 800 mg every day to be effective in 89% and 87% of patients, respectively (Pappas et al. Response charges have been larger in those with acute pulmonary disease (100%, two of two patients) and disseminated disease (71%), than in those with the continual pulmonary illness (46%). These success rates have been decrease than the success rates reported for itraconazole (Negroni et al. Again, fewer patients responded to fluconazole induction remedy in contrast with itraconazole (74% vs. For sufferers within the examine persevering with on fluconazole maintenance remedy of four hundred mg every day, the rate of relapse was excessive (31%), with relapsefree survival of only 53% after one year. Together, these studies show that fluconazole is simply moderately efficient for treatment of histoplasmosis. Dermatophytosis Open-label, noncomparative trials inspecting fluconazole (150 mg weekly for 1�5 weeks) for the remedy of fungal dermatophytosis have documented success rates of approximately 70�95%. Of the 20 sufferers enrolled in one open trial, a complete clinical treatment price was documented in 95% of patients with tinea corporis or tinea cruris and in 70% of sufferers with tinea pedis (Montero-Gei and Perera, 1992). The efficacy fee for patients with tinea corporis or tinea cruris receiving 4 doses (100%) was greater than the rate for patients receiving only two (75%) or three doses (67%). Another open-label examine documented a short-term scientific success fee of 92% and a long-term clinical remedy fee of 88% in 95 patients with tinea corporis, tinea cruris, or cutaneous candidiasis (Suchil et al. Relapse occurred in 7% of sufferers with tinea corporis and 14% of sufferers with tinea cruris. In a third trial of 71 patients with tinea pedis receiving a imply of three fluconazole doses, shortterm scientific treatment was documented in 74% and long-term cure was observed for 77% of sufferers (Del Aguila et al. These research show efficacy of fluconazole within the remedy of tinea infections, with higher success rates seen for tinea corporis and tinea cruris compared with tinea pedis. Although efficacious overall, fluconazole achieves lesser treatment rates than griseofulvin or terbinafine for dermatophtoses but has the advantage of being simpler to administer. A potential, non-blinded, cross-sectional study of the comparative efficacies of terbinafine, griseofulvin, and fluconazole was undertaken in kids aged 12 years with tinea 7h. Histoplasmosis Open-label trials have demonstrated only moderate efficacy for fluconazole within the therapy of histoplasmosis (SharkeyMathis et al. However, just like the study of blastomycosis, research with itraconazole have advised enhanced efficacy for this azole. Fluconazole is recommended instead just for patients illiberal of itraconazole when the patient is clinically steady. A total of 75 patients (25 in every treatment group) who completed the designated remedy protocol have been included in the last evaluation. Cure rates of 96%, 88%, and 84% have been achieved with griseofulvin, terbinafine, and fluconazole, respectively (Grover et al. The efficacy of single oral doses of fluconazole 400 mg or itraconazole 400 mg was in contrast in a randomized trial (Partap et al. Relapse related to optimistic Malassezia furfur cultures was extra widespread in sufferers handled with itraconazole (60% vs. Single-dose fluconazole outperformed itraconazole for therapy of pityriasis versicolor. Onychomycosis Several trials have examined the efficacy of fluconazole for the therapy of onychomycosis. Fluconazole appears to be less effective than both itraconazole or terbinafine for the remedy of dematophyte nail infections. Fluconazole was compared with these agents in an open-label research of fifty patients diagnosed with dermatophyte distal subungal onychomycosis (Arca et al. Patients received fluconazole (150 mg weekly), itraconazole (200 mg bid for 1 week of each month), or terbinafine (250 mg day by day for a total of three months). At the 6-month analysis, the clinical response price was significantly lower in patients treated with fluconazole (38%) than in those that had obtained both itraconazole (78%) or terbinafine (81%). Mycologic response was additionally lower in fluconazole-treated patients (31%) than in itraconazole- (61%) and terbinafine- (65%) handled patients. Of 133 sufferers, patients had been subdivided into receiving fluconazole one hundred fifty mg once weekly, itraconazole continual remedy, itraconazole pulse remedy, terbinafine 250 mg every day, and terbinafine plus ciclopirox 8% lacquer-cure charges at 48 weeks have been ninety two. The decision to offer a number of therapies must be evaluated of the dangers and benefits of various therapies together with the potential for drug�drug interactions. Although a number of antifungal brokers could additionally be used in the treatment of coccidioidomycosis, major therapy most often consists of an azole, either fluconazole or itraconazole. The place of the newer azoles antifungal therapy of coccidioidomycosis is mentioned in section 3, Antifungal medication. In most instances fluconazole is most popular because of its superior bioavailability, comparable efficacy, and lack of unwanted facet effects and drug�drug interactions. In one observational research of one hundred and five patients with main pulmonary coccidioidomycosis (Ampel et al. The results of this study emphasize the need for extended follow-up for sufferers with severe illness requiring remedy. The second examine was a 24-week, observational research among 36 patients with gentle to average coccidioidomycosis (Blair et al. If a decision to initiate antifungal therapy for main coccidioidomycosis is made, the dose of fluconazole ought to be 400 mg per day to be continued for a minimal of 6 months (Ampel, 2015). Treatment of diffuse and/or persistent fibrocavitary pneumonia with fluconazole requires high doses (800�1000 mg/ day) for at least 1 year (Catanzaro et al. In diffuse overwhelming disease, some specialists recommend initial therapy with amphotericin B (either deoxycholate amphotericin B at 0. The frequency of amphotericin B infusions may be decreased over time because the patient clinically improves. However, for less extreme disease, significantly these not requiring hospitalization, oral fluconazole alone at doses starting from four hundred to 2000 mg/day is an inexpensive therapy (Galgiani et al. Patients with skeletal an infection responded twice as often to itraconazole as to fluconazole. The dosage used in the first open label clinical trial was 400 mg/day where 37 of 47 (79%) evaluable patients responded to therapy (Galgiani et al. Some physicians choose the utilization of higher doses and start treatment with 800 or 1000 mg/day of fluconazole (Galgiani et al.
Purchase solian 100 mg with amexChanges in the treatment responses to artesunate�mefloquine on the northwest border of Thailand throughout thirteen years of steady deployment treatment goals for anxiety generic solian 100mg overnight delivery. Population pharmacokinetics of mefloquine in military personnel for prophylaxis in opposition to malaria an infection throughout field deployment treatment zit generic 100 mg solian. Influence of hemodialysis on plasma concentration�time profiles of mefloquine in two patients with end-stage renal disease: a prophylactic drug monitoring examine medications used to treat bipolar disorder generic solian 50mg with mastercard. Neurological symptoms quit drinking purchase solian 100mg line, cardiovascular and metabolic results of mefloquine in healthy volunteers: a double-blind, placebo-controlled trial. Quantitative evaluation of antimalarial activity in vitro by a semiautomated microdilution technique. Drug-induced pneumonia associated with hemizygote glucose-6-phosphate-dehydrogenase deficiency. Inhibition of human neutrophil protein kinase C activity by the antimalarial drug mefloquine. Quinoline antimalarials: mechanisms of action and resistance and prospects for model new agents. Role of cytochrome P450 3A within the metabolism of mefloquine in human and animal hepatocytes. Effects of mefloquine on Ca2+ uptake by crude microsomes of rabbit skeletal muscle. Thermodynamics of partitioning of the antimalarial drug mefloquine in phospholipid bilayers and bulk solvents. Comparison of the exercise in vitro of mefloquine and two metabolites against Plasmodium falciparum. In vitro susceptibility and genetic variations for chloroquine and mefloquine in Plasmodium falciparum isolates from Thai�Myanmar border. Plasma concentrations of sulfadoxine�pyrimethamine and of mefloquine throughout common long run malaria prophylaxis. Enantioselective pharmacokinetics of mefloquine during long-term intake of the prophylactic dose. Gene amplification of the multidrug resistance 1 gene of Plasmodium vivax isolates from Thailand, Laos and Myanmar. Mefloquine disposition in normals and in patients with extreme Plasmodium falciparum malaria. A comparability of the pharmacokinetics of mefloquine in wholesome Thai volunteers and in Thai patients with falciparum malaria. Plasmodium falciparum: position of absolute stereochemistry in the antimalarial activity of synthetic amino alcohol antimalarial agents. Efficacy and safety of mefloquine, artesunate, mefloquine�artesunate, and praziquantel against Schistosoma haematobium: randomized, exploratory open-label trial. A doubleblind comparative clinical trial of mefloquine and chloroquine in symptomatic falciparum malaria. In vitro monitoring of Plasmodium falciparum susceptibility to artesunate, mefloquine, quinine and chloroquine in Cambodia: 2001�2002. Studies of mefloquine bioavailability and kinetics utilizing a steady isotope method: a comparability of Thai sufferers with falciparum malaria and healthy Caucasian volunteers. The enantioselective binding of mefloquine enantiomers to P-glycoprotein determined using an immobilized P-glycoprotein liquid chromatographic stationary section. Inhibition of volumeregulated and calcium-activated chloride channels by the antimalarial mefloquine. Morphological results and tegumental alterations induced by mefloquine on schistosomula and adult flukes of Schistosoma mansoni. Carbenoxolone and mefloquine suppress tremor within the harmaline mouse mannequin of essential tremor. Mefloquine selectively will increase asynchronous acetylcholine launch from motor nerve terminals. Successful treatment of refractory disseminated Mycobacterium avium complicated infection with the addition of linezolid and mefloquine. Mefloquine antimalarial prophylaxis in pregnancy: dose finding and pharmacokinetic examine. Treatment of multidrug-resistant Plasmodium falciparum malaria with 3-day artesunate-mefloquine mixture. Mefloquine prophylaxis prevents malaria during pregnancy: a double-blind, placebo-controlled research. In vitro synergy and enhanced murine brain penetration of saquinavir coadministered with mefloquine. User acceptability patterns for mefloquine and doxycycline malaria chemoprophylaxis. Plasmodium falciparum pfmdr1 amplification, mefloquine resistance and parasite health. The in vitro susceptibilities of Ghanaian Plasmodium falciparum to antimalarial medication. Unexpected frequency, duration and spectrum of antagonistic occasions after therapeutic dose of mefloquine in healthy adults. Ketoconazole will increase plasma concentrations of antimalarial mefloquine in wholesome human volunteers. Genetic linkage of pfmdr1 with food vacuolar solute import in Plasmodium falciparum. The absorption, distribution, and excretion in mice of the antimalarial mefloquine, erythro-2,8-bis(trifluoromethyl)-alpha-(2-piperidyl)-4-quinolinemethanol hydrochloride. A randomized trial of artesunate� mefloquine versus artemether�lumefantrine for remedy of uncomplicated Plasmodium falciparum Malaria in Mali. Pregnancy and fetal outcomes after exposure to mefloquine in the pre- and periconception period and through pregnancy. Mefloquine tolerability throughout chemoprophylaxis: give attention to antagonistic event assessments, stereochemistry and compliance. Tolerability of malaria chemoprophylaxis in non-immune travelers to sub-Saharan Africa: multicentre, randomised, double blind, 4 arm research. Urinary excretion of mefloquine and some of its metabolites in African volunteers at steady state. The susceptibility of the malarial parasite Plasmodium falciparum to quinoline-containing drugs is correlated to the lipid composition of the infected erythrocyte membranes. Simple and inexpensive fluorescence-based method for high-throughput antimalarial drug screening. Effectiveness of 5 artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial.

Order solian 50 mg fast deliveryIn all besides one of many pharmacokinetic-evaluable topics (1/187; <1%) posaconazole Cavg exposures of 500 ng/ml or higher were achieved when administered without regard to meals medicine grand rounds cheap solian 50 mg otc. A robust correlation was found between the posaconazole trough focus and noticed Cavg values (R2 = 0 medicine used for adhd discount solian 50mg fast delivery. A rising body of evidence suggests relatively broad patient-to-patient variability in peak serum concentrations for the suspension formulation of posaconazole (Krishna et al medications zolpidem order solian 50 mg overnight delivery. These variations seem unbiased of age symptoms 2dpo discount 100mg solian mastercard, renal operate, hepatic function, sex, ethnicity, or physique mass (Sansone-Parsons et al. The variability is presumably a results of erratic absorption in some patient populations. For instance, bone marrow transplant recipients had been discovered to have 50% decrease serum posaconazole ranges in contrast with wholesome controls (Ullmann et al. Although the pattern measurement was small, this distinction is theorized to be a results of a shortened terminal half-life in this subset of patients (17 hours compared with 29 hours). The presence of mucositis was additionally advised to alter absorption in this affected person cohort. In addition, diarrhea has been proven to alter posaconazole serum concentrations (Lebeaux et al. A small study examined the serum degree of posaconazole in 12 pediatric patients, ages 8�17, who received 800 mg posaconazole every day in divided doses for therapy of invasive fungal infections (Krishna et al. At steady-state, related imply posaconazole plasma levels were measured for pediatric (776 ng/ml) and adult (817 ng/ml) patients. Although vital interpatient variability was seen, these outcomes suggest that older pediatric patients require a dose just like adults to preserve the identical posaconazole steadystate concentrations. Issues relating to the utility of posaconazole therapeutic drug monitoring are discussed beneath (5c, Clinically necessary pharmacokinetic and pharmacodynamic features). Drug distribution Posaconazole is extensively distributed all through the body, with good extravascular tissue penetration. In two pharmacokinetic research of the oral suspension formulation, the apparent quantity of distribution (Vd) for posaconazole ranged from 343 to 1341 L (Courtney et al. In wholesome volunteers given a single 400-mg dose of posaconazole (suspension formulation), peak plasma ranges reached 654 ng/ml (Krieter et al. However, while the posaconazole tablets yield considerably improved drug exposures, the terminal-phase halflife (t1/2), the apparent complete physique clearance, and the apparent V/F of both oral formulations are comparable (Krishna et al. In two part 1B research on the intravenous formulation, patients in danger for invasive fungal infections acquired either 200 mg or 300 mg posaconazole qd, after an preliminary loading dose of 200 mg or 300 mg bid on day 1. In one case report, an excellent distribution of posaconazole into the vitreous has been noticed (Sponsel et al. Data regarding its penetration into cerebrospinal fluid is inconsistent and very restricted (Calcagno et al. In one patient with Fusarium endophthalmitis and keratitis receiving posaconazole (800 mg qd and 2-hourly topical ocular applications), posaconazole concentrations within the vitreous and the plasma had been zero. This diploma of vitreal penetration (21%) is lower than that described for voriconazole (38%) and fluconazole (28�75%), however greater than that reported for itraconazole (10%) (Ashley et al. However, renal parenchymal drug concentrations are just like plasma concentrations, suggesting the potential utility of posaconazole within the therapy of invasive fungal an infection involving the kidney. Regarding the oral suspension formulation, therapeutic drug monitoring is beneficial by many experts as a outcome of its low bioavailability, unpredictable plasma concentrations, and broad inter-individual variability associated this formulation (Dolton et al. However, restricted information are available on the utility of posaconazole therapeutic drug monitoring (Andes et al. Monitoring was undertaken in an openlabel trial of posaconazole for the remedy of refractory aspergillosis. In this patient cohort, a statistically and clinically significant relationship between posaconazole serum focus and treatment end result was noticed. Efficacy was best (75% response rate) in patients with imply 2850 Posaconazole steady-state Cavg concentrations > 1. However, since only a small subset of sufferers was recognized with an infection, no significant relationship between serum drug ranges and infections could presumably be established (Krishna et al. Notably, a therapeutic drug monitoring reference laboratory found that 70% of 202 serum samples from sufferers receiving the oral suspension formulation contained less than seven-hundred ng/ml of posaconazole (Thompson et al. These information suggest that therapeutic drug monitoring must be performed when administering the oral suspension formulation. Because the delayed-release pill and the intravenous formulation are extra reliable in archiving the target common steady-state concentrations, therapeutic drug monitoring many not be needed for those formulations. Excretion Posaconazole undergoes minimal metabolism (15%) and is primarily excreted unchanged into the feces (Krieter et al. In healthy volunteers receiving a single 400-mg dose of [14C] posaconazole, 14% of radioactivity was recovered within the urine (Krieter et al. Examination of posaconazole metabolites in wholesome volunteers demonstrates that the majority of metabolites characterize glucuronide conjugates quite than oxidative metabolic merchandise (Krieter et al. Hence it has the capacity for drug interactions through these mechanisms (see Table 157. Dosages of these immunosuppressants should be decreased and drug levels must be rigorously monitored. In a small examine of six sufferers receiving posaconazole, cyclosporine exposure elevated and required cyclosporine dosage discount of 14�29% in four patients (Sansone-Parsons et al. Like different triazoles, co-administration with drug inducers can decrease posaconazole serum levels. Posaconazole also has the potential for drug interactions by interference of P-glycoprotein drug transporters. In vitro research counsel that posaconazole, although weak in comparison to ketoconazole and itraconazole, is a substrate and inhibitor of P-glycoprotein (Merck Sharp & Dohme, 2009; Sansone-Parsons et al. The role of P-glycoprotein in posaconazole pharmacokinetics and drug interactions ought to be further investigated. Gastrointestinal symptoms (diarrhea, nausea, and vomiting) have been reported in 15% of patients receiving posaconazole for persistent febrile neutropenia or refractory invasive fungal an infection (Ullmann et al. Therefore, monitoring of liver perform tests is really helpful earlier than and during treatment. Hence, in patients with reasonable to severe renal dysfunction (creatinine clearance (CrCl) < 50 ml/min) posaconazole oral formulations, as an alternative of the intravenous formulation, should be used- unless the profit outweights the danger. In very young dogs (dosed from 2�8 weeks of age), receiving the intravenous formulation, an increased incidence of brain ventricle enlargement was observed. Juvenile canines (4 days to 9 months of age) receiving an oral formulation of posaconazole showed no neurologic, behavioral or developmental abnormalities (Merck Sharp & Dohme, 2009). A randomized trial evaluating posaconazole suspension to standard azole prophylaxis (fluconazole or itraconazole) in 602 neutropenic patients present process chemotherapy for 2852 Posaconazole acute myelogenous leukemia or myelodysplastic syndrome discovered fewer invasive fungal infections in patients receiving posaconazole (2%) than in these receiving either fluconazole or itraconazole (8%) (Cornely et al. Invasive aspergillosis was diagnosed in fewer sufferers receiving posaconazole (1%) than normal azole prophylaxis (7%).
Order solian 50 mg on lineDrug distribution Mebendazole can be measured in a quantity of tissues symptoms 6 year molars cheap solian 100mg on-line, most notably the liver medications diabetes order solian 50mg amex, and in echinococcal cysts medications medicaid covers purchase solian 50mg with amex, the place the concentrations correlated well with the free mebendazole plasma concentrations four hours after dosing (Luder et al shinee symptoms mp3 best 100mg solian. The focus of mebendazole-related material in liver tissue is considerably higher than in fatty tissue or parasite materials from patients with cystic echinococcosis. The cerebrospinal fluid focus in one affected person receiving 200 mg/kg every day was eight. There is critical interindividual variability in bioavailability and metabolism of mebendazole. In a gaggle of patients treated with mebendazole for hydatid disease with a 10-mg/kg oral dose, the half-life of the drug ranged from 2. Case reports exist of erratic migration of Ascaris lumbricoides, delicate gastrointestinal upset, transient stomach pain, and diarrhea (Chavarria et al. When the drug has been given at high doses for extended periods for therapy of cystic or alveolar echinococcal disease (50 mg/kg for 3�4 weeks), unwanted effects reported have included extreme abdominal pain, elevated transaminase levels, central nervous system disturbance (vertigo, headache), alopecia, and bone marrow despair, together with neutropenia (Miskovitz and Javitt, 1980; Levin et al. Clinically necessary pharmacokinetic and pharmacodynamic options There are few knowledge to instantly correlate the clinical exercise of mebendazole with its pharmacokinetic and pharmacodynamic parameters. Excretion A massive proportion (about half) of the absorbed dose is excreted within the urine as conjugated and unidentified metabolites, as estimated using tracer doses (Dawson et al. The two major metabolites are the amide hydrolysis product (2-amino-5-benzoylbenzimidazole), and the product of ketone reduction, (methyl-5[-hydroxybenzyl]-2-benzimidazole carbamate). Conjugates of those and an extra metabolite of mebendazole have been found in bile along with a conjugate of the mother or father drug. However, these metabolites comprise solely a small proportion of drug metabolites excreted in the urine, accounting for only 0. The main unconjugated urinary metabolite is the product of each ketone discount and hydrolysis, (2-amino-5(6) [-hydroxybenzyl]benzimidazole), and accounts for 87% of unconjugated material in urine (0. Risks in pregnancy In rats, mebendazole is teratogenic at doses of forty mg/kg (Van den Bossche et al. In a survey of one hundred seventy girls who took mebendazole in the first trimester of being pregnant, the speed of congenital abnormalities, fetal loss, or neonatal demise was not significantly higher than that observed within the basic inhabitants (Cowden and Hotez, 2000). In a second series of 112 exposures, just one congenital malformation was observed (hand malformation) (Cowden and Hotez, 2000). In a 3rd large retrospective study sponsored by the World Health Organization, over 7000 Sri Lankan girls who had by accident taken mebendazole during being pregnant had been studied (de Silva et al. However, there was a pattern toward the next incidence of main congenital defects amongst those who took the drug through the first trimester, with a price of 2. In one other placebo-controlled research undertaken in Peru amongst women within the second and third trimesters of pregnancy, no distinction from placebo was noticed other than a helpful effect on birth weight, whereby the frequency of very low delivery weight was decreased amongst these handled with mebendazole. Efficacy has been invariably 95�100%, and is accompanied by high reductions within the number of eggs excreted in the feces. Recent studies from several countries have proven that the originally reported high efficacy persists, with high treatment and egg discount charges (Levecke et al. Trichuriasis Trichuris trichiunra inhabits the lower intestine, and the poorer cure charges which have been seen with many anthelmintics have been attributed to decrease drug exposures and dilution effects in the colon. Early research of both mebendazole and albendazole advised that efficacy was low, however more modern data suggest that mebendazole, both as a single dose or with the usual one hundred mg twice-daily regimen, could additionally be more practical than first believed. In two latest giant studies covering seven international locations, efficacy to a single 500-mg dose ranged from 40�70%, whereas the identical dose given for three days was simpler (71%) (Levecke et al. It is necessary to note, however, that in distinction to other intestinal helminth infections, anthelmintic efficacy is inversely associated to the preliminary egg load, though that is less pronounced with mebendazole than albendazole (Levecke et al. The normal dose used is either 100 mg twice daily for 3 days or, extra just lately, as a single dose of 500 mg. Early medical studies on the efficacy of mebendazole had been small and used methodologies that might at present be thought-about insufficient. Although knowledge from many of these early studies support the efficacy of mebendazole, only studies printed since 1980 have been included right here to support doses or levels of efficacy or to indicate where there have been changes from the older materials. Ascariasis Ascaris lumbricoides is likely considered one of the easier intestinal helminths to treat, and it is extremely sensitive to most anthelmintics, together with the benzimidazoles. Hookworm the usual dose for therapy of hookworm an infection is both 100 mg twice day by day for three days or 500 mg as a single Table 201. Condition Enterobius Ascaris, hookworm, Trichuris Strongyloides Trichostrongylus Echinococcus granulosus Echinococcus Multilocularis Capillaria philippinensis (P. The most intensive longitudinal surveillance of anthelmintic exercise has been performed in Tanzania, the place there has been continuous use of mebendazole over many years. However, since mebendazole is apparently simpler than albendazole towards trichuriasis, and blended infections of intestinal helminths are widespread, the choice of drug must be made on the premise of the infecting species. A single case report exists documenting the efficacy of mebendazole in trichostrongyliasis, utilizing a dose of 100 mg twice every day for three days (Farahmandian et al. Benzimidazole anthelmintics are the drugs of selection over the older drugs, though these are still effective. Single 100-mg doses of mebendazole are nicely tolerated by all ages and are efficient with remedy rates of close to one hundred pc. Treatment of the entire household, repeated 2�4 weeks later, is necessary to ensure treatment of the entire household group. Recent research have confirmed the continued efficacy of mebendazole and the necessity for remedy of the whole household (Nikolic et al. Cystic and alveolar echinococcosis Until the mid-1970s when mebendazole became available, there was no therapy for echinococcosis other than surgery. Because absorption of mebendazole is poor, and rapid biotransformation to inactive metabolites happens within the liver, very giant doses are wanted. Doses used have ranged from 20 mg/kg to as high as 50 mg/kg/day, with the occasional patient receiving up 200 mg/kg in cases of severe or in depth illness (Messaritakis et al. The early research advised that mebendazole was efficient and doubtless parasitostatic, although at this stage solely extreme, inoperable cases were studied (Muller et al. Importantly, a selection of medical studies have in contrast the efficacy of mebendazole and albendazole (which has an lively systemic metabolite). In the most important of those, almost 500 sufferers who had been treated and followed up for a couple of years in Rome are reported (Franci et al. This collection, utilizing solely continuous therapy with both drugs, documents the acute and long-term responses to treatment of patients with cystic Echinococcus in all kinds of internet sites. Although a significant medical response to mebendazole was noticed in 56% of topics treated, the response was considerably greater (82%) with albendazole. Strongyloidiasis Infection with Strongyloides stercoralis occurs mostly in moist tropical regions, but can be present in returning travelers. A small number of veterans of conflicts in endemic areas develop continual infections that can be tough to treat. Benzimidazole anthelmintics have been used for a couple of years and have been reported to be efficient and properly tolerated. The usual dose of mebendazole is one hundred mg twice daily for three days, which is adequate to kill the grownup worms.
Cheap solian 50 mg otcIn one research treatment nerve damage buy discount solian 50 mg on-line, sulconazole nitrate 1% cream utilized twice daily for 14 days to sufferers with pyoderma caused by Streptococcus pyogenes and Staphy lococcus spp 247 medications solian 50mg with mastercard. Following 4 weeks of therapy treatment thesaurus discount 100mg solian, scientific cure was observed in 75% of sufferers treated with neticonazole alone versus 52% of sufferers handled with neticonazole plus dressing medicine qd generic 50mg solian with amex. Comparative trials of terconazole 80mg pessaries with clo trimazole vaginal tablets revealed equal mycologic effi cacy (95% and 85%) and a extra speedy onset of symptomatic reduction for terconazoletreated girls (Kjaeldgaard and Lars son, 1985). In comparative research, clinical and mycologic efficacy of oxiconazole was equal to miconazole cream, econazole cream, clotrimazole cream, bifonazole cream, naf tifine, terbinafine, and tolnaftate cream (Gip, 1984; Wagner Tioconazole 1% or 2% cream is an effective remedy for dermatomycoses, including tinea corporis, tinea cruris, and tinea pedis, with clinical and mycologic remedy rates of 75�95% (Kuokkanen, 1982; Smith et al. Tioconazole 1% cream utilized two or three times day by day for 2 weeks was considerably more effective than 1% clotrimazole cream within the treatment of cutaneous candidiasis, with medical and mycologic cure rates of 71% (Clissold and Heel, 1986). Clinical makes use of of the medication 2895 mazole, econazole, and miconazole, but tioconazole has equal efficacy to these brokers at a minimum, and may have superior efficacy to econazole and clotrimazole (Clissold and Heel, 1986). Comparative efficacy of naftifine, oxiconazole, and terbinafine in short-term treatment of tinea pedis. Flutrimazole 1% dermal cream within the therapy of dermatomycosis: a multicentre, doubleblind, randomized, comparative clinical trial with bifonazole 1% cream. Clinical experience with fenticonazole 2% formulation in the remedy of dermatomycoses and pityriasis versicolor. Three day therapy of vulvovaginal candidiasis with econazole: a multicentric research comprising 996 cases. Treatment of tinea corporis or tinea cruris with bifonazole 1% gel: an open, multicenter examine. Three-day remedy of vaginal candidiasis with clotrimazole vaginal tablets and econazole ovules: a multicentre comparative study. Sertaconazole: an antifungal agent for topical treatment of superficial candidiasis. Comparative medical trial of bifonazole answer versus selenium sulphide shampoo in the remedy of pityriasis versicolor. Surgical treatment of canine nasal aspergillosis by rhinotomy combined with enilconazole infusion and oral itraconazole. Sertaconazole: a review of its use within the management of superficial mycoses in dermatology and gynaecology. Superficial dermatomycoses worldwide: multinational therapy expertise with a combination of isoconazole nitrate and diflucortolone valerate. Sertaconazole nitrate quickly achieves excessive concentrations within the stratum corneum with extended retention time. A double-blind randomized comparative trial: eberconazole 1% cream versus clotrimazole 1% cream twice every day in Candida and dermatophyte skin infections. Comparison of flutrimazole site-release cream (1, 2 and 4%) with placebo site-release vaginal cream. Tioconazole 2% cream within the remedy of Trichomonas vaginalis or blended vaginal infections. The retention of isoconazole in the pores and skin after once or twice every day application of 1% isoconazole nitrate cream (Travogen) over a 14-day period. Bifonazole 1% gel in the therapy of superficial dermatophytoses and erythrasma of the toes and groin. Controlled study of fluconazole within the prevention of fungal infections in neutropenic patients with haematological malignancies and bone marrow transplant recipients. Pharmacokinetics and tolerance of sertaconoazole in man after repeated percutaneous administration. Ultrastructural modifications in onychomycosis during therapy with bifonazole/urea ointment. An open, multicentre assessment of the effectiveness of bifonazole in the remedy of tinea (pityriasis) versicolor. Response of tinea corporis-cruris and tinea (pityriasis) versicolor to as quickly as daily topical treatment with bifonazole cream: a safety and efficacy examine. Oxiconazole within the therapy of vaginal candidiasis: single dose versus 3-day treatment with econazole. Assessment of bifonazole 1% answer within the eradication of organisms causing tinea (pityriasis) versicolor. An overview of topical antifungal therapy in dermatomycoses: a North American perspective. Efinaconazole 10% answer nail answer: a brand new topical therapy with broad antifungal exercise for onychomycosis monotherapy. The advantages of topical mixture therapy within the therapy of inflammatory dermatomycoses. Systemic absorption and persistence of tioconazole in vaginal fluid after insertion of a single 300 mg tioconazole ovule. Oxiconazole nitrate: pharmacology, efficacy, and security of a brand new imidazole antifungal agent. Single-blind comparative trial of short-term therapy with terconazole versus clotrimazole vaginal tablets in vulvovaginal candidiasis. A evaluation of its antimicrobial activity and therapeutic use in superficial mycoses. Croconazole: a new broad spectrum agent in the treatment of fungal skin infections. Topical antiinflammatory properties of flutrimazole, a brand new imidazole antifungal agent. Comparative efficacy and tolerance of 1% bifonazole cream and bifonazole cream automobile in sufferers with tinea versicolor. Therapeutic efficacy and safety of the new antimycotic sertaconazole in the remedy of pityriasis versicolor. Systemic absorption of 3H-fenticonazole after vaginal administration of 1 gram in sufferers. Pharmacokinetic studies following systemic and topical administration of radiolabeled bifonazole in man. Therapeutic efficacy and safety of the new antimycotic sertaconazole in the therapy of cutaneous dermatophytosis. Biological disposition and percutaneous absorption of bifonazole in animals and man. A comparability of butoconazole nitrate cream with econazole nitrate cream for the remedy of vulvovaginal candidiasis. Long-term outcomes in dogs with sinonasal aspergillosis treated with intranasal infusions of enilconazole. Treatment with bifonazole shampoo for seborrhea and seborrheic dermatitis: a randomized, double-blind examine. An evaluation of butoconazole nitrate 2% website release vaginal cream (Gynazole-1) in comparison with fluconazole 150 mg tablets (Diflucan) in the time to aid of signs in patients with vulvovaginal candidiasis. Usefulness of lanoconazole (Astat) cream in the treatment of hyperkeratotic sort tinea pedis. Comparative research of monotherapy and mixture therapy with 10% urea ointment (Pastaron).
References - Williams G, Foyle A, White D, et al. Intravascular T-cell lymphoma with bowel involvement: case report and literature review. Am J Hematol. 2005;78(3):207-211.
- Ballhausen H, Li M, Ganswindt U, et al: Shorter treatment times reduce the impact of intra-fractional motion : a real-time 4DUS study comparing VMAT vs. step-and-shoot IMRT for prostate cancer, Strahlenther Onkol 194(7):664n674, 2018.
- Benson RC, Swanson SK, Farrow GM: Relationship of leukoplakia to urothelial malignancy, J Urol 131:507n511, 1984.
- Endo M, Yamashita T, Jin HY, et al: Detection of human papillomavirus type 16 in bowenoid papulosis and nonbowenoid tissues, Int J Dermatol 42:474n476, 2003.
|